Protecting the Protector Boosts Plant Oil Content
Scientists demonstrate new genetic strategy for preventing breakdown of plant oils needed for biofuels and other products
February 8, 2024
Brookhaven Lab biologist Sanket Anaokar is the first author on a paper describing ways to get plants to accumulate more oil by protecting a protein that keeps oil from being broken down. The scientists demonstrated increased oil accumulation in tobacco leaves (foreground), but the strategy could potentially be applied in oil-crop plants such as sorghum (background). (Kevin Coughlin/Brookhaven National Laboratory)
UPTON, NY—Biologists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have demonstrated a new way to boost the oil content of plant leaves and seeds. As described in the journal New Phytologist, the scientists identified and successfully altered key portions of a protein that protects newly synthesized oil droplets. The genetic alterations essentially protect the oil-protector protein so more oil can accumulate.
“Implementing this strategy in bioenergy or oil crop plants could help meet the growing demand for biodiesel fuel and/or nutritionally important plant oils,” said Brookhaven Lab biochemist John Shanklin, chair of the Lab’s Biology Department, who led the research.
Shanklin’s team has been working for years to boost plant oil accumulation, particularly in parts of plants such as leaves that generally don’t make a lot of oil. These vegetative tissues typically account for most of the plant biomass. Boosting their ability to accumulate oil would substantially increase the biomass energy content. And since vegetable oils are key feedstocks for making biodiesel, the strategy could turn crop plants into green factories for producing sustainable fuels.
Push, pull, protect
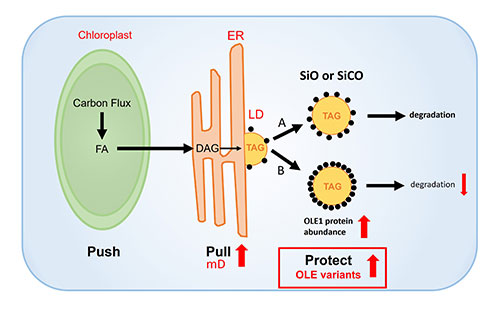
Model showing the use of modified OLE1 in PUSH-PULL-PROTECT strategy for increasing oil accumulation in plant cells. The strategy combines modifications to push carbon into the production of triacylglycerol (TAG), a form of oil that is stored in lipid droplets (LD), using an efficient mouse diacylglycerol O-acyltransferase 2 gene (mD). In this study, the scientists explored ways to modify oleosin, a protein that protects lipid droplets from degradation. A. With oleosin variants SiO or SiCO, there is still significant oil degradation. B. Variant OLE1 is more resistant to breakdown, so it provides more protection and results in decreased oil degradation. (Sanket Anaokar/Brookhaven National Laboratory)
Much of the Brookhaven team’s focus has been implementing genetic strategies that biochemically push plant cells to make more oil and pull that newly synthesized oil into storage in lipid droplets—rather than shuttling it into building new plant parts.
“But once oil is made, it can be broken down, and the level of accumulation is the balance between synthesis and breakdown,” Shanklin explained.
So, the scientists have also used a third approach: cranking up production proteins that protect lipid droplets from being degraded.
One such protective protein naturally made by plants is known as oleosin. Oleosin becomes embedded in the oil-droplet membrane, blocking access to enzymes called lipases that initiate the breakdown of oil.
“We and others typically ramp up levels of this small protein to protect the lipid droplets,” Shanklin said.
But oleosin itself can be degraded, limiting its effectiveness. So, in the new work, Shanklin and his team set out to find a way to protect the oil protector.
“This was a complicated puzzle that lead author Sanket Anaokar worked creatively to solve,” Shanklin said. Anaokar is a Brookhaven Lab research associate in the Center for Advanced Bioenergy and Bioproducts Innovation (CABBI) program, a DOE-funded Bioenergy Research Center led by the University of Illinois Urbana-Champaign.
Left: Study coauthors Yuanxue Liang, John Shanklin, and Sanket Anaokar holding Arabidopsis plants used to confirm altered gene expression and oil accumulation. Right: A closeup of Arabidopsis leaves and seed pods. (Kevin Coughlin/Brookhaven National Laboratory)
Deleting degradation signals
“We reasoned that if we could identify and remove the parts of oleosin that the degradation enzymes recognize—the degradation ‘signals’—we could get oleosin to stick around and enhance oil accumulation,” Anaokar said.
Using clues from other groups that had used a different approach to tackle this problem, the scientists engineered variants of the oleosin protein and tested their effects in tobacco leaves. The team initially designed the variants to change all the amino acids hypothesized to be involved in the degradation of oleosin. Then, they reverted the mutations back one at a time and looked for the biggest changes in oil accumulation. In the end, this allowed them to identify a few key mutations that made oleosin significantly more resistant to breakdown.
“These changes made the variant forms of oleosin accumulate to higher levels—which in turn more efficiently protected the oil, so the oil levels also rose,” Shanklin said.
Plants expressing the most successful mix of genetic modifications accumulated 54% more oil in their leaves and 13% more in their seeds compared to unmodified plants.
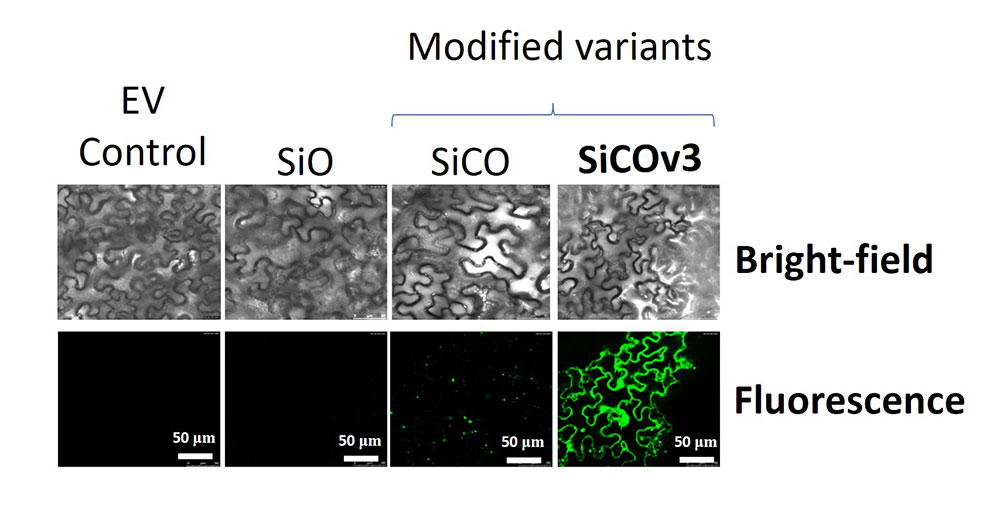
Confocal microscopy images of tobacco leaves showing the relative abundance of oleosin protein. The most successful variant (far right) resulted in significantly greater oleosin abundance (green fluorescence) compared to other variants and empty vector (EV) control. This finding was consistent with the observation of greater oil accumulation in this same variant and the role of oleosin as a lipid-droplet protector. Bright-field images represent the tissue that was used for imaging. (Sanket Anaokar/Brookhaven National Laboratory)
Serendipitous surprise
One surprising finding was that the modifications to protect oil droplets did not have negative effects on plant growth or the ability of the seeds to germinate. This was surprising because plant seeds need to break down stored oil to fuel germination and the early stages of seedling growth —that is, until the plant has established itself and grown enough leaves for photosynthesis to kick in and fuel further growth.
“We initially had concerns that preventing oil breakdown during seed development would inhibit this ‘establishment’ process,” Shanklin said. “But we discovered that establishment is unaffected by the oleosin variants. This tells us that, during early growth, the plant uses another mechanism for breaking down oil so seedlings can get access to its stored energy.
“We don’t yet know what that process is, but it allows us to use oleosin variants to increase oil accumulation in vegetative tissue and seeds without impairing seedling growth,” Shanklin said.
This research was supported by the DOE Office of Science, via CABBI. It was initiated with support from the Renewable Oil Generated with Ultra-productive Energycane (ROGUE) program, also led by the University of Illinois. In addition to the biochemical and genetic studies in plants, the team used confocal microscopy at Brookhaven Lab’s Center for Functional Nanomaterials (CFN), a DOE Office of Science user facility.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
Related Links