Moving away from lithium – towards sodium?
One of the simplest ideas would be to replace lithium with sodium. This element is anything but rare: sodium chloride is found in seawater and is accessible all over the world. But this is about it when it comes to advantages. Since a sodium ion is about 50 percent larger than a lithium ion, the sodium cathode materials show poorer electrochemical cycling performance. For instance, sodium cobalt oxide, which is the sodium ion analog of lithium cobalt oxide (i.e., the conventional cathode material of the lithium-ion battery) can sustain much fewer charging cycles. This basically eliminates the cost advantage. There are also problems on the opposite side of the battery, with the anode material. Graphite (as in lithium-ion batteries) is not suitable for sodium batteries, because it does not store enough sodium ions. Tests with cheap tin, antimony or phosphorus showed good charge storage properties, but when charging, the anode expands percent to about three times its original volume. This impairs the mechanical stability: When subjected to shocks, the bloated material can easily disintegrate, and the battery would be damaged. With phosphorus anodes it gets even worse: When charging, sodium phosphide (Na3P7) is formed in the anode, which, together with water, produces phosphine: an extremely toxic gas that leads to respiratory arrest; this is how metal phosphides act as a rat poison. Hard to believe anyone would like to have such a battery, fully charged with solar power, in their cellars.
How about magnesium?
In the chemical periodic table magnesium resides next to sodium. It is a small, light atom and can transfer two electrons at once. Magnesium is inexpensive and non-toxic. Could it be used to make batteries? On the anode side of the battery, magnesium has advantages: You don’t need graphite (as in lithium-ion batteries), instead you can use a magnesium foil as anode. However, the small, double-charged magnesium ion brings drawbacks on the cathode side. The high electrical charge on a small diameter leads to high electrical attraction forces. For example, the ion slips into a lattice of cobalt oxide only with great force, and if it is stuck there, it is difficult to extract it again. Anyone who tries to do so by force – i.e. with higher voltages – runs the risk of triggering oxidation and reduction processes in the chemical components of the battery, thereby destroying them. Besides of that the high voltages, which are necessary to reverse the chemical process makes these batteries very inefficient. As a consequence, such batteries cannot be charged at high speed and can only be used in a small voltage range if they are to last a long time.
Aluminium Graphite Batteries
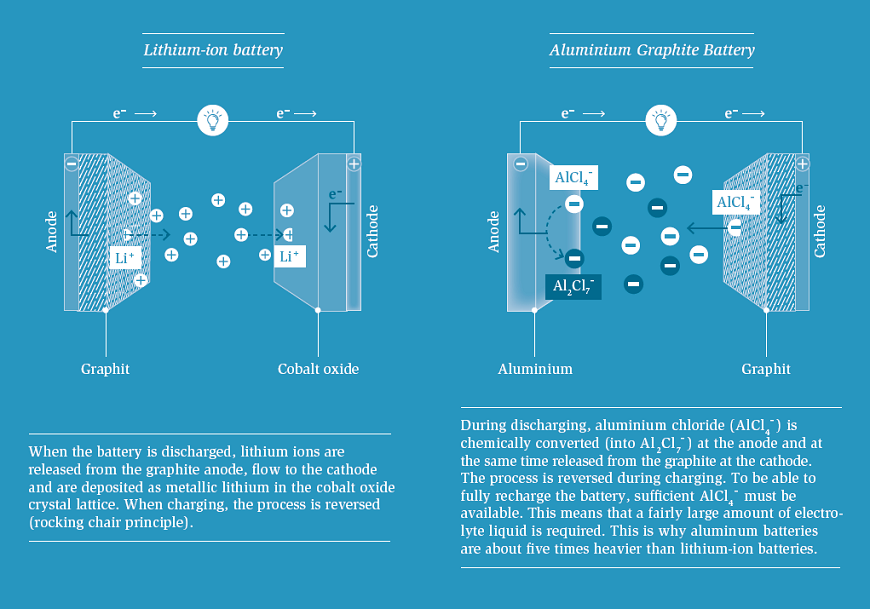
If we walk further along in the periodic table, aluminium is next. This metal is also available in large quantities, non-toxic and inexpensive. It can transfer three electrons at once. Similar to the magnesium battery, the anode is easy to build: An alumnium foil is all you need.
But the rest of the aluminium battery operates fundamentally different from lithium-ion batteries: Lithium-ion batteries employ the “rocking chair” principle. When discharging, the lithium ions migrate from the anode to the cathode; when charging, they mi grate back. By contrast, in an aluminium graphite battery, there is no one- directional motion of Al3+ ions from the positive to the negative electrodes. Instead, Al species are “consumed” from the chloroaluminate ionic liquid – the electrolyte – during the charging of the battery by both electrodes. The electrolyte hence plays a double function: It provides aluminium, which is deposited in the form of metal on the anode, and acts as the source of AlCl4- ions needed for the intercalation into the positive graphite electrode during charging. The amount of electrolyte is, therefore, decisive for the capacity of the battery. Thus, aluminium graphite batteries will be about five times heavier than comparable Li-ion batteries due to their chemical operating principle. Apart from that, the graphite cathode expands to more than twice its original volume during each charging process and shrinks again during discharging. This means: In any case, such batteries need flexible envelopes and protective casings with sufficient space to “breathe”. The expansion and shrinkage also affects the shock resistance and long-term stability. This may need remedies in the construction of the cathode.
New battery management
An additional challenge will be the charging algorithm for non-lithium-ion batteries. The team led by Kravchyk and Kovalenko discovered that the performance of an alumnium-graphite electrode could be increased by up to 25 percent through skillful, step-by-step charging. A research group working in Taiwan, China, USA and Germany found that such electrodes are significantly more powerful when cooled to –10 degrees Celsius. These results illustrate that a completely new battery management system, i.e. new sensors, chargers and algorithms, has to be developed in parallel with these chemically entirely different battery types. It is still unclear which of the battery technologies described above will prevail and one day can replace lithium- ion batteries in some areas. In their assessment the researchers emphasize that none of these technologies can compete with lithium-ion batteries in terms of energy density. This is not likely to change in future. These types of alternative batteries are therefore only suitable for applications, in which electricity is to be stored as cheaply as possible and the focus is on sustainable production of the batteries.
More applied research is needed
Therefore, a lot of work remains to be done by research groups the world over if alternative battery technologies are to outperform the good, old lithium-ion battery. Kostiantyn Kravchyk and Maksym Kovalenko call for a more holistic approach. “With their lab experiments, researchers often only prove the feasibility of an idea – the cost of all necessary components and the estimated total weight of the entire battery system are mostly neglected,” says Kravchyk. However, it is exactly these parameters that are crucial for a possible commercialization. “They should thus be given a much greater consideration in research than to date.” Despite their somewhat sobering study, Kostiantyn Kravchyk will continue to re-search alternative storage batteries in the future. “Systems using graphite as cathode material are interesting. We were already able to show that the swelling and shrinking of the cathode material is a problem that can be overcome.” Together with his colleagues, he is now exploring “semi-solid” graphite electrodes that can last a long time and at the same time transmit electricity very well.
Original post https://alertarticles.info



