The Science
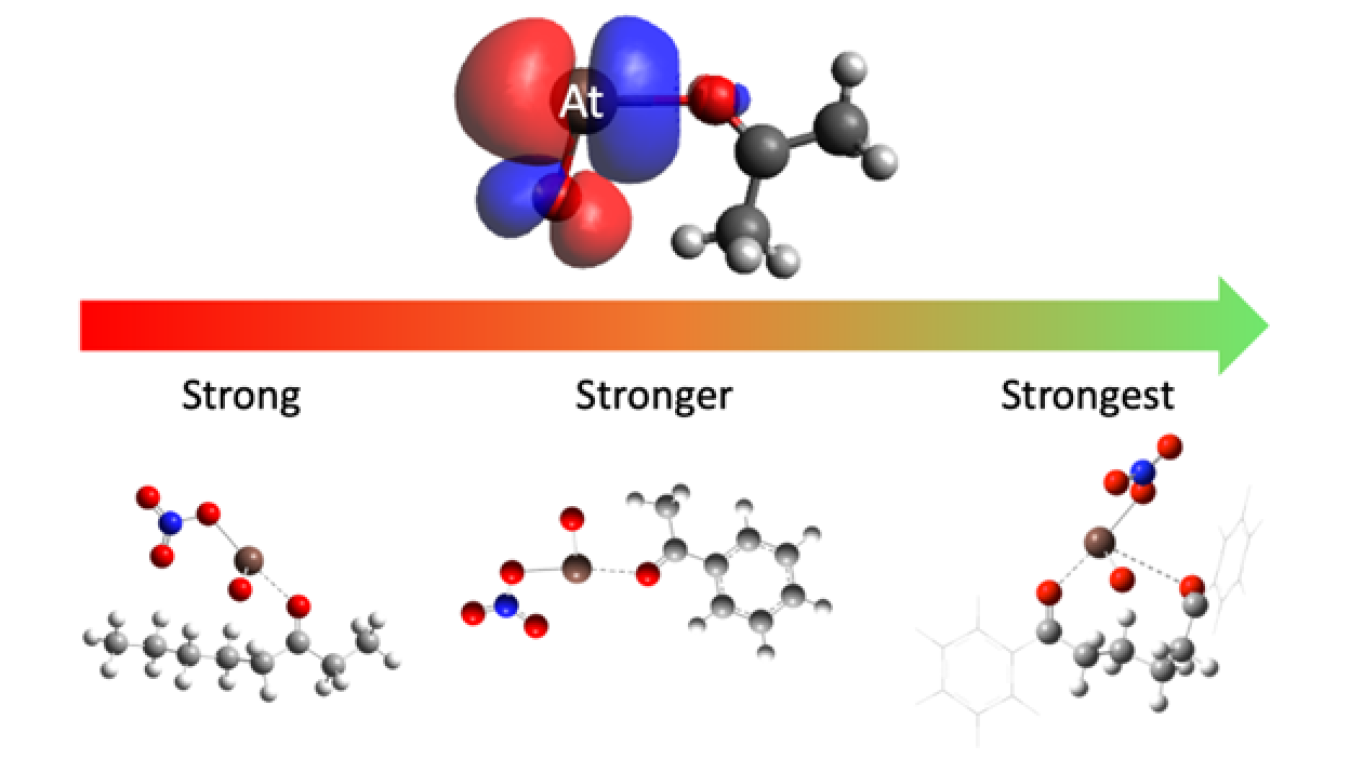
Astatine is the least abundant element on Earth, and all of its known isotopes have a half-life of less than 8 hours. One astatine isotope, astatine-211 (At-211), emits alpha particles and shows promise as a cancer therapy. However, very little is known about astatine’s chemical interactions. In this research, scientists have discovered a new bonding interaction with a class of chemicals known as ketones. The interaction means scientists can tune the bond strength between ketones and At-211 by adjusting the type of ketone used. This allows scientists to control for how tightly the At-211 is held to the ketone. The discovery has the potential to improve cancer therapy drugs by linking At-211 to cancer targeting molecules.
The Impact
Researchers are increasingly interested in targeted alpha therapy (TAT) drugs for cancer treatment. TAT is a process in which an alpha emitting atom, capable of causing high damage at a short range, is attached to molecules that target cancer cells. At-211 shows promise for TAT because it emits one alpha particle and has a short half-life (7.2 hours). Despite At-211 not being widely available, scientists have used the isotope in clinical trials, including the treatment of malignant brain tumors, ovarian cancer, and a current study treating blood cancer. The new discovery of the tunable At-211 – ketone interaction opens the door for a new class of TAT drugs.
Summary
Making At-211 requires a nuclear reaction resulting from bombarding a metal plate of bismuth with alpha-particles using a specialized particle accelerator. The Texas A&M K150 cyclotron is one of about 22 facilities worldwide capable of such a reaction. Building on previous work, researchers at Texas A&M University and the University of Alabama at Birmingham discovered a new, tunable chemical interaction of At-211 with a class of chemicals known as ketones. They explored the tunability of this interaction by making subtle changes to the ketone.
The results indicate that changing the ketone can either strengthen or weaken the bonding interaction with At-211. Improving the bond strength of At-211 will allow At-211 to be held tightly and linked to cancer targeting molecules. This opens the door for developing a new class of improved At-211 based TAT drugs.
Contact
Sherry Yennello
Texas A&M University
[email protected]
Jon Burns
University of Alabama at Birmingham
[email protected]
Funding
This work was supported by the Department of Energy (DOE), the DOE Isotope Program managed by the Office of Isotope R&D and Production, Texas A&M University through the Bright Chair in Nuclear Science, and the Texas A&M System National Laboratories Office through a collaboration with Los Alamos National Laboratory. This work was also enabled by the Texas A&M Nuclear Solutions Institute. One of the researchers acknowledges support from the College of Arts and Sciences and the O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham. Another researcher acknowledges financial support from the Welch Foundation and the National Science Foundation.
Publications
Burns, J. D., et al. Complexation of Astatine(III) with Ketones: Roles of NO3(-) Counterion and Exploration of Possible Binding Modes. Inorganic Chemistry 61, 31 (2022). [DOI: 10.1021/acs.inorgchem.2c00085]
Scraped from https://www.sourcearu.com




