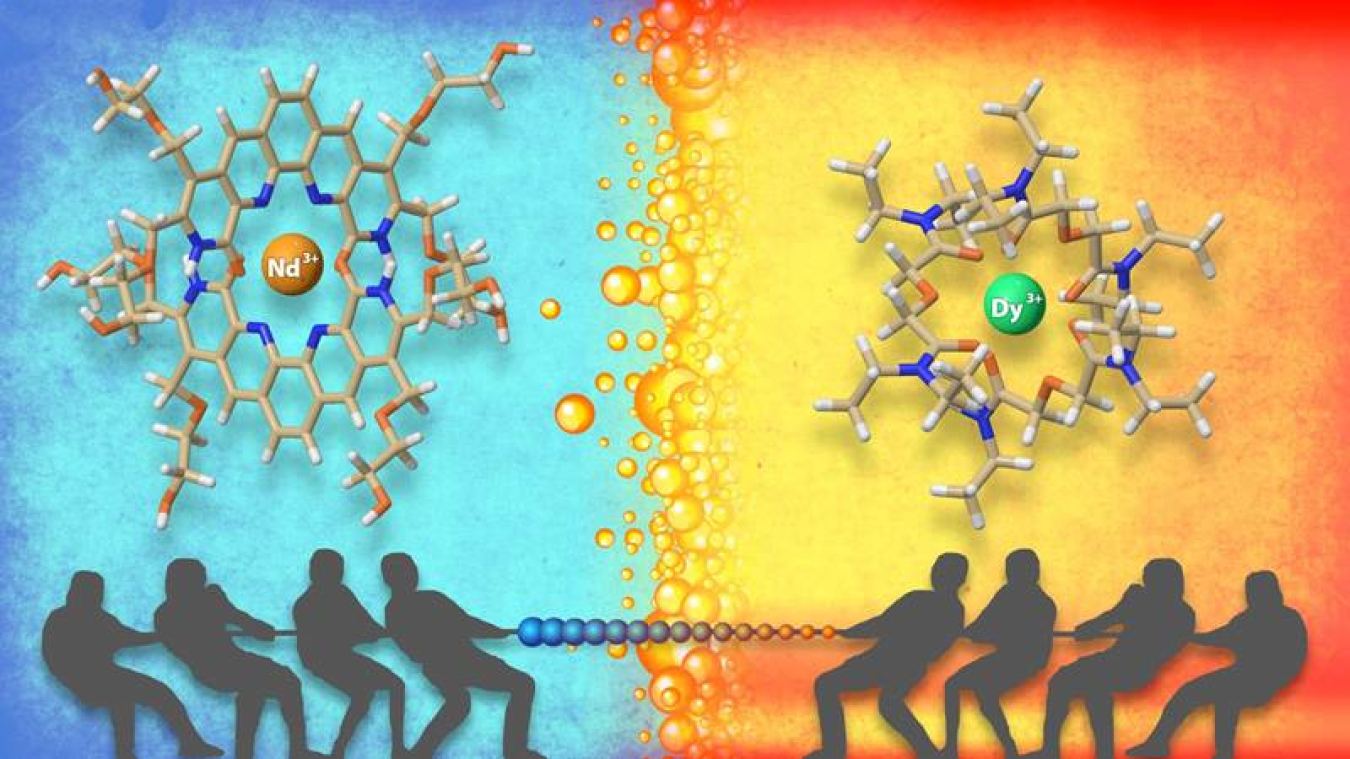
The Science
The metals called lanthanides have valuable properties for clean energy technologies such as electric vehicles and wind turbines and for many other applications. These elements include several critical materials. In nature, lanthanides are often found mixed together. Industry must separate them to take advantage of their individual properties. But conventional approaches to this separation are time consuming and costly and generate waste. Scientists have developed an efficient new method that can be tailored to select specific lanthanides. The technique combines two substances. One is water-loving and catches lighter lanthanides, while the other prefers oil and grabs heavier lanthanides.
The Impact
Blending an oil-loving and a water-loving compound together to pull specific valuable elements from a chemical mixture is feasible on an industrial scale. Scaled up, the process would allow for smaller equipment, less use of chemicals, and less waste production. This would make the new process more efficient and environmentally friendly than conventional methods.
Summary
The most challenging and expensive aspect of making pure rare earth materials — the 14 lanthanides as well as yttrium and scandium — for clean energy technologies is separating individual rare earth elements from one another. Scientists from Oak Ridge National Laboratory combined two types of organic substances: one water loving, and the other oil loving. These organic substances have preferences for different rare earth elements. For instance, one interacts strongly with the lighter rare earth elements, while the other prefers the heavier ones.
The scientists tested this technique using two different liquids that do not mix — oil and water. In water, they dissolved the water-loving substance; in oil, they added the oil-loving one. They found that the two-substance approach helped separate the lightest and heaviest rare earth elements better than the one-substance method applied previously. They used various methods to study how these organic chemicals and rare earth elements interact. The outcome was valuable information about how the process works and insights concerning how the separation system could be further improved.
Contact
Santa Jansone-Popova
Oak Ridge National Laboratory
[email protected]
Funding
This work was supported by the Department of Energy Office of Science, Office of Basic Energy Sciences, Separation Science program and Materials Chemistry program.
Publications
Jansone-Popova, S., et al., Size Selective Ligand Tug of War Strategy to Separate Rare Earth Elements. JACS Au, 3, 584-591 (2023). [DOI: 10.1021/jacsau.2c00671]
Related Links
Tug-of-war strategy supercharges lanthanide separation, Oak Ridge National Laboratory news
Scraped from https://www.sourcearu.com




