The Science
Communities of microbes that live in ocean sediments can consume methane. In oxygen-deprived sediments these microbes form clusters, called aggregates, that can have deposits of silica on their surfaces. It is not clear if these silica deposits result from the activity of methane consuming aggregates, or if their formation is unrelated to biological processes. This study showed that silica rich nanoparticles formed on cell aggregates in culture, even when the chemical composition of water should have prevented it. It suggests that microbial activity is involved in their formation.
The Impact
Microbes called anaerobic methanotrophic archaea form communities with sulfate reducing bacteria. These communities can consume methane without the need for oxygen. Processes associated with these microbes can create silica deposits that appear to entomb the communities. Silica deposition helps to preserve aggregates in the geological record. This discovery connects the way microbes process methane with the transformation of silica. It will aid scientists in identifying fossil evidence for this ancient microbial activity in the rock record.
Summary
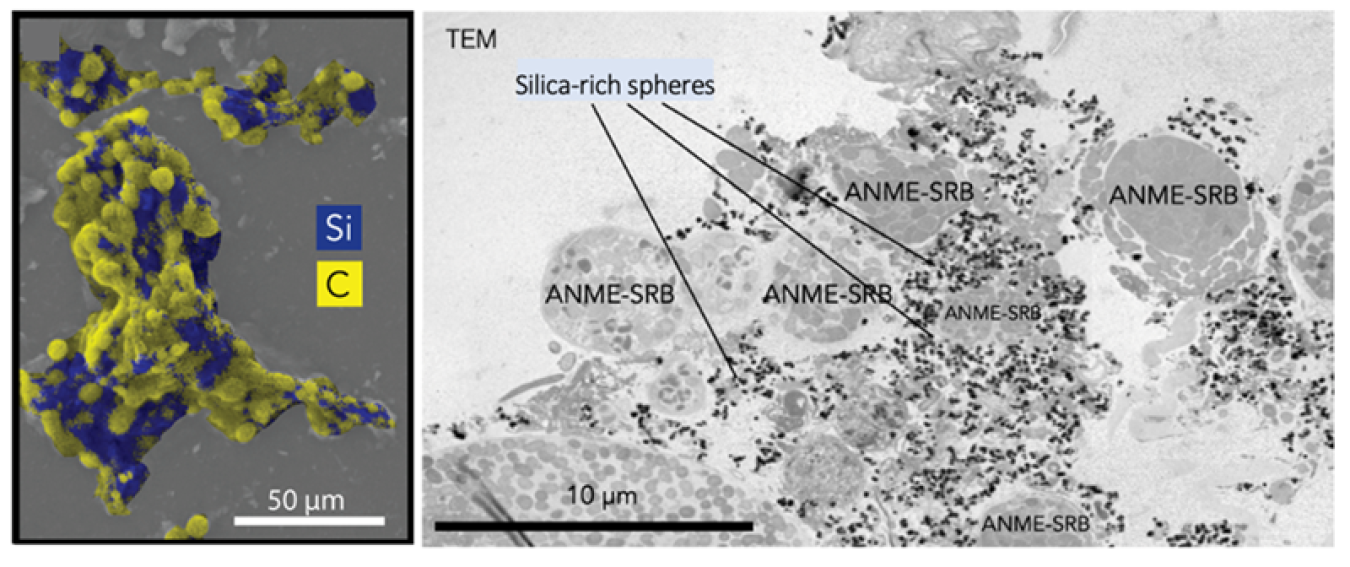
Minerals can preserve microbial biosignatures in the rock record, where they serve as diagnostic indicators of ancient microbial processes like methane oxidation. In modern methane-rich environments, partnerships between anaerobic methane-oxidizing archaea (ANME) and sulfate-reducing bacteria (SRB) link the microbial cycling of carbon, sulfur, iron, and nitrogen through their metabolic activities. However, their potential impact on the silica cycle has been largely overlooked. This study offers new insights on how ANME-SRB consortia contribute to biologically induced silica precipitation. Researchers used enrichment cultures of ANME-SRB consortia to investigate this phenomenon under controlled laboratory conditions and observed the formation of silica-rich spheres (~200 nm) within the exopolymer-rich matrix of the consortia. These silica-rich particles formed in artificial seawater media that was undersaturated with respect to crystalline and amorphous silica, suggestive of biological influence on their formation. Mineral stability modeling suggested this form was likely an amorphous form of kaolinite clay associated with the organic matrix.
Using correlative fluorescence in situ hybridization, scanning electron microscopy with energy dispersive X-ray spectroscopy, and nanoscale secondary ion mass spectrometry, researchers expanded this study to the field, testing for the occurrence of Si-rich minerals associated with uncultured anaerobic methane-oxidizing consortia in authigenic carbonates and sediments. These analyses revealed silica-rich phases on consortia like those observed in the enrichment cultures. Microbially-induced precipitation not only contributes to silica transformation in methane-rich environments, but also may enhance the preservation of ANME-SRB consortia as microfossils. The intimate association between these consortia and silica offers a potential mineralogical signature for fossilized methane oxidizing communities, helping to identify them in ancient methane cycling ecosystems.
Contact
Victoria J. Orphan
California Institute of Technology
[email protected]
Funding
Funding for this work was provided by the Department of Energy Office of Science, the National Science Foundation, a Gordon and Betty Moore Foundation Marine Microbiology Investigator grant, the Simons Collaboration for the Origin of Life, the Center for Environmental Microbial Interactions at California Institute of Technology, a Schlanger Ocean Drilling Fellowship, and the CIFAR Earth 4D program.
Publications
Osorio-Rodriguez, D., et al., Microbially induced precipitation of silica by anaerobic methane-oxidizing consortia and implications for microbial fossil preservation. Proceedings of the National Academy of Sciences 120, 51 (2023). [DOI: 10.1073/pnas.23021561]
Scraped from https://www.sourcearu.com




